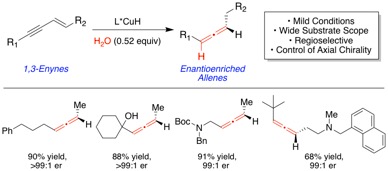
The general enantioselective synthesis of axially chiral disubstituted allenes from prochiral starting materials remains a long-standing challenge in organic synthesis. Here, we report an efficient enantio- and chemoselective copper hydride- catalyzed semi-reduction of conjugated enynes to furnish 1,3-disubstituted allenes using water as the proton source. This protocol is sufficiently mild to accommodate an assortment of functional groups including keto, ester, amino, halo, and hydroxyl groups. Additionally, applications of this method for the selective synthesis of mono-deuterated allenes and chiral 2,5-dihydropyrroles are described.