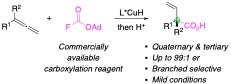
We report a method to prepare α-chiral carboxylic acid derivatives, including those bearing all-carbon quaternary centers, through an enantioselective CuH-catalyzed hydrocarboxylation of allenes with a commercially available fluoroformate. A broad range of heterocycles and functional groups on the allenes were tolerated in this protocol, giving enantioenriched α-quaternary and tertiary carboxylic acid derivatives in good yields with exclusive branched regioselectivity. The synthetic utility of this approach was further demonstrated by derivatization of the products to afford biologically important compounds, including the antiplatelet drug indobufen.