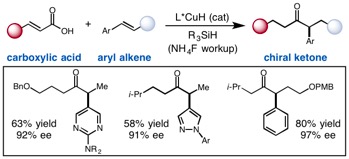
The direct asymmetric copper hydride (CuH)-catalyzed coupling of α,β-unsaturated carboxylic acids to aryl alkenes is reported to access chiral α-aryl dialkyl ketones. A variety of substrate substitution patterns, sensitive functional groups and heterocycles are tolerated in this reaction, which significantly expands the range of accessible products compared to existing hydroacylation methodology. Although mechanistic studies are ongoing, we propose that CuH-catalyzed silylation of unsaturated acids occurs to access a uniquely effective acyl electrophilic coupling partner.The direct asymmetric copper hydride (CuH)-catalyzed coupling of α,β-unsaturated carboxylic acids to aryl alkenes is reported to access chiral α-aryl dialkyl ketones. A variety of substrate substitution patterns, sensitive functional groups and heterocycles are tolerated in this reaction, which significantly expands the range of accessible products compared to existing hydroacylation methodology. Although mechanistic studies are ongoing, we propose that CuH-catalyzed silylation of unsaturated acids occurs to access a uniquely effective acyl electrophilic coupling partner.
