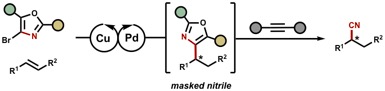
The enantioselective hydrocyanation of olefins represents a conceptually straightforward approach to prepare enantiomerically enriched nitriles. These, in turn, comprise or are intermediates in the synthesis of many pharmaceuticals and their synthetic derivatives. Herein, we report a cyanide-free dual Pd/CuH-catalyzed protocol for the asymmetric Markovnikov hydrocyanation of vinyl arenes and the anti-Markovnikov hydrocyanation of terminal olefins in which oxazoles function as nitrile equivalents. After an initial hydroarylation process, the oxazole substructure was deconstructed using a [4+2]/retro-[4+2] sequence to afford the enantioenriched nitrile product under mild reaction conditions.The enantioselective hydrocyanation of olefins represents a conceptually straightforward approach to prepare enantiomerically enriched nitriles. These, in turn, comprise or are intermediates in the synthesis of many pharmaceuticals and their synthetic derivatives. Herein, we report a cyanide-free dual Pd/CuH-catalyzed protocol for the asymmetric Markovnikov hydrocyanation of vinyl arenes and the anti-Markovnikov hydrocyanation of terminal olefins in which oxazoles function as nitrile equivalents. After an initial hydroarylation process, the oxazole substructure was deconstructed using a [4+2]/retro-[4+2] sequence to afford the enantioenriched nitrile product under mild reaction conditions.
