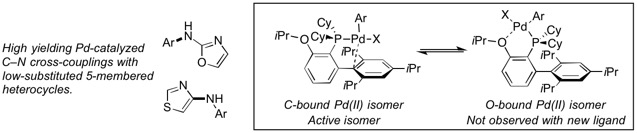
Mechanistic Insight Leads to a Ligand That Facilitates the Pd-Catalyzed Formation of 2-(Hetero)Arylaminooxazoles and 4-(Hetero)Arylaminothiazoles
“Mechanistic Insight Leads to a Ligand That Facilitates the Pd-Catalyzed Formation of 2-(Hetero)Arylaminooxazoles and 4-(Hetero)Arylaminothiazoles”, Angew. Chem. Int. Ed., 2017, 56, 10569-10572.