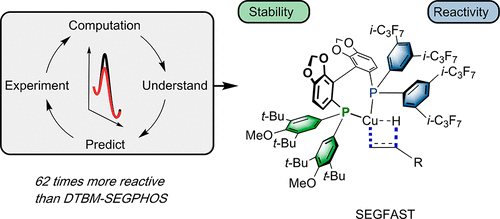
Using a mechanically guided ligand design approach, a new ligand (SEGFAST) for the CuH-catalyzed hydroamination reaction of unactivated terminal olefins has been developed, providing a 62-fold rate increase over reactions compared to DTBM-SEGPHOS, the previous optimal ligand. Combining the respective strengths of computational chemistry and experimental kinetic measurements, we were able to quickly identify potential modifications that lead to more effective ligands, thus avoiding synthesizing and testing a large library of ligands. By optimizing the combination of attractive, non-covalent ligand-substrate interactions and the stability of the catalyst under the reaction conditions, we were able to identify a finely-tuned hybrid ligand that greatly enables accelerated hydrocupration rates with unactivated alkenes. Moreover, a modular and robust synthetic sequence was devised, which allowed for practical, gram-scale synthesis of these novel hybrid ligand structures.Using a mechanically guided ligand design approach, a new ligand (SEGFAST) for the CuH-catalyzed hydroamination reaction of unactivated terminal olefins has been developed, providing a 62-fold rate increase over reactions compared to DTBM-SEGPHOS, the previous optimal ligand. Combining the respective strengths of computational chemistry and experimental kinetic measurements, we were able to quickly identify potential modifications that lead to more effective ligands, thus avoiding synthesizing and testing a large library of ligands. By optimizing the combination of attractive, non-covalent ligand-substrate interactions and the stability of the catalyst under the reaction conditions, we were able to identify a finely-tuned hybrid ligand that greatly enables accelerated hydrocupration rates with unactivated alkenes. Moreover, a modular and robust synthetic sequence was devised, which allowed for practical, gram-scale synthesis of these novel hybrid ligand structures.
