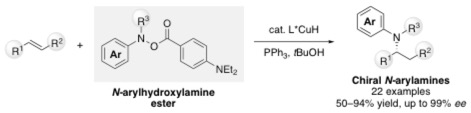
Despite significant recent progress in the copper-catalyzed enantioselective hydroamination chemistry, the synthesis of chiral N-arylamines, which are frequently found in natural products and pharmaceuticals, has not been realized. Initial experiments with N-arylhydroxylamine ester electrophiles were unsuccessful and, instead, their reduction, in the presence of copper hydride (CuH) catalysts, was observed. Herein, we report key modifications of our previously reported hydroamination protocols that lead to broadly applicable conditions for the enantioselective net addition of secondary anilines across the double bond of styrenes, 1,1-disubstituted olefins, and terminal alkenes. NMR studies suggest that suppression of the undesired reduction pathway as the basis for the dramatic improvements in yield under the reported protocol.
