Palladium-Mediated Arylation of Lysine in Unprotected Peptides
“Palladium-Mediated Arylation of Lysine in Unprotected Peptides”, Angew. Chem. Int. Ed., 2017, 56, 3177-3181.
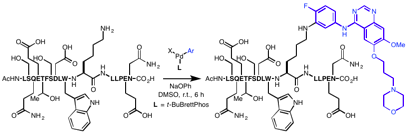
A mild method for the arylation of lysine in an unprotected peptide is presented. In the presence of a preformed biarylphosphine-supported Pd(II)-aryl complex and weak base, lysine amino groups underwent C–N bond formation at room temperature. The process generally exhibited high selectivity for lysine over other amino acids containing nucleophilic side chains and was applicable to the conjugation of a variety of organic compounds, including complex drug molecules, with an array of peptides. Lastly, this method was also successfully applied to the formation of cyclic peptides via macrocyclization.