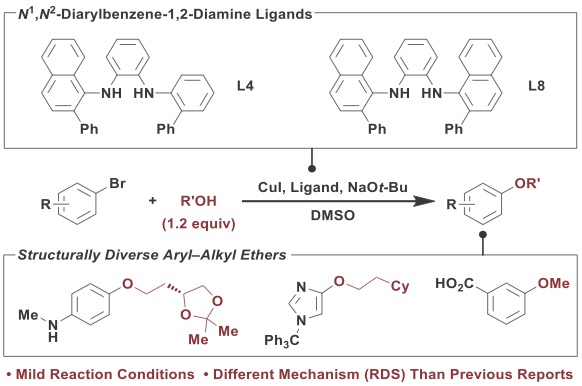
We disclose the development of a Cu-catalyzed C–O coupling method utilizing a new N1,N2-diarylbenzene-1,2-diamine ligand, L8. Under optimized reaction conditions, structurally diverse aryl and heteroaryl bromides underwent efficient coupling with a variety of alcohols at room temperature using an L8-based catalyst. Notably, the L8-derived catalyst exhibited enhanced activity when compared to the L4-based system previously disclosed for C–N coupling, namely the ability to functionalize aryl bromides containing acidic functional groups. Mechanistic studies demonstrate that C–O coupling utilizing L8•Cu involves rate-limiting alkoxide transmetallation, resulting in a mechanism of C–O bond formation that is distinct from previously described Pd-, Cu-, or Ni-based systems. This lower energy pathway leads to rapid C–O bond formation; a 7-fold increase relative to what is seen with other ligands. The results presented in this report overcome limitations in previously described C–O coupling methods and introduce a new ligand that we anticipate may be useful in other Cu-catalyzed C–heteroatom bond-forming reactions.
