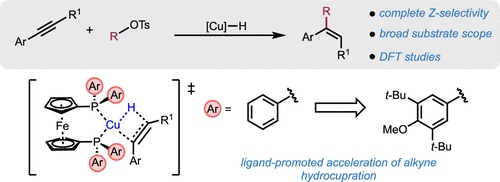
Alkenes are ubiquitous in organic chemistry, yet many classes of alkenes remain challenging to access by current synthetic methodology. Herein, we report a copper hydride-catalyzed approach for the synthesis of Z-configured trisubstituted alkenes with high stereo- and regioselectivity via alkyne hydroalkylation. A DTBM-dppf-supported Cu catalyst was found to be optimal, providing a substantial increase in product yield compared to reactions conducted with dppf as the ligand. DFT calculations show that the DTBM substitution leads to the acceleration of alkyne hydrocupration through combined ground and transition state effects related to preventing catalyst dimerization and enhancing catalyst–substrate dispersion interactions, respectively. Alkyne hydroalkylation was successfully demonstrated with methyl and larger alkyl tosylate electrophiles to produce a variety of (hetero)aryl-substituted alkenes in moderate to high yields with complete selectivity for the Z stereochemically configured products. In the formation of the key C–C bond, computational studies revealed a direct SN2 pathway for alkylation of the vinylcopper intermediate with in situ-formed alkyl iodides.
