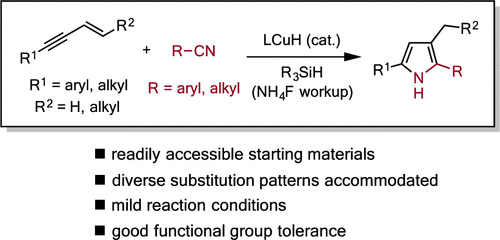
Herein, we describe an efficient method to prepare polysubstituted pyrroles via a copper-hydride (CuH)-catalyzed enyne-nitrile coupling reaction. This protocol accommodates both aromatic and aliphatic substituents and a broad range of functional groups, providing a variety of N-H pyrroles in good yields and with high regioselectivity. We propose that the Cu-based catalyst promotes both the initial reductive coupling and subsequent cyclization steps. Density functional theory (DFT) calculations were performed to elucidate the reaction mechanism.Herein, we describe an efficient method to prepare polysubstituted pyrroles via a copper-hydride (CuH)-catalyzed enyne-nitrile coupling reaction. This protocol accommodates both aromatic and aliphatic substituents and a broad range of functional groups, providing a variety of N-H pyrroles in good yields and with high regioselectivity. We propose that the Cu-based catalyst promotes both the initial reductive coupling and subsequent cyclization steps. Density functional theory (DFT) calculations were performed to elucidate the reaction mechanism.
