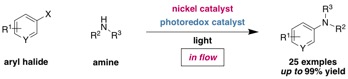
We report a visible light-mediated flow process for C–N cross-coupling of (hetero)aryl halides with a variety of amine coupling partners through the use of a photoredox/nickel dual catalyst system. Compared to the method in batch, this flow process enables a broader substrate scope, including less-activated (hetero)aryl bromides and electron-deficient (hetero)aryl chlorides, and significantly reduced reaction times (10 to 100 minutes). Furthermore, scale up of the reaction, demonstrated through the synthesis of tetracaine, is easily achieved, delivering the C–N cross-coupled products in consistently high yield of 84% on up to a 10 mmol scale.