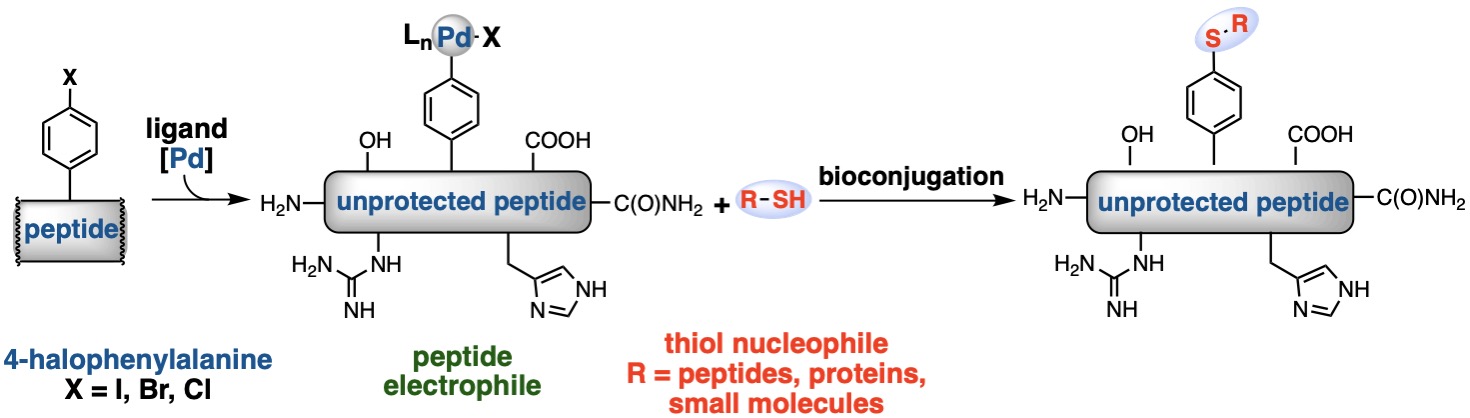
The synthesis of palladium oxidative addition complexes derived from unprotected peptides is described. Incorporation of 4-halophenylalanine into a peptide during solid phase peptide synthesis allows for subsequent oxidative addition at this position upon treatment with a palladium precursor and suitable ligand. The resulting palladium−peptide complexes are solid, storable, water-soluble, and easily purified via high-performance liquid chromatography. These complexes react with thiols in aqueous buffer, offering an efficient method for bioconjugation. Using this strategy, peptides can be functionalized with small molecules to prepare modified aryl thioether side-chains at low micromolar concentrations. Additionally, peptide−peptide and peptide−protein ligations are demonstrated under dilute aqueous conditions.
