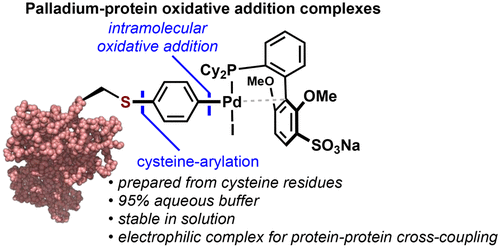
Few chemical methods exist for the covalent conjugation of two proteins. We report the preparation of site-specific protein–protein conjugates that arise from the sequential cross-coupling of cysteine residues on two different proteins. The method involves the synthesis of stable palladium–protein oxidative addition complexes (Pd-protein OACs), a process that converts nucleophilic cysteine residues into an electrophilic S-aryl-Pd-X unit by taking advantage of an intramolecular oxidative addition strategy. This process is demonstrated on proteins up to 83 kDa in size and can be conveniently carried out in water and open to air. The resulting Pd-protein OACs can cross-couple with other thiol-containing proteins to arrive at homogeneous protein–protein bioconjugates.
